Developmental Stages in Human Embryos
Go to Stage: Intro 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Stage 10
Page 93Approximately 1.5–3 mm in length
Approximately 22 ± 1 postovulatory days
Characteristic feature: 4–12 pairs of somites
SUMMARY
External: 4–12 pairs of somites; fusion of neural folds is imminent or in progress; the optic sulcus may have appeared; pharyngeal arch 1 begins to be visible on the surface.
Internal: the cardiac loop is appearing; the laryngeotracheal sulcus develops; the intermediate mesoderm becomes visible.
SIZE AND AGE
The chorion generally has a diameter of 8–15 mm. The greatest length of the embryo, although not of great informational value (Bartelmez and Evans, 1926), is usually 1.5–3 mm.
The age is approximately 22 postovulatory days. A brief review of stage 10 was published by Heuser and Corner (1957), and a detailed investigation of this stage was undertaken by Müller and O'Rahilly (1985), who provided graphic reconstructions. Both of these publications contain an appropriate bibliography.
EXTERNAL FORM
The criterion for stage 10 is the presence of 4–12 pairs of somites. This stage is particularly important because, during it,the neural tube first begins to be formed from the neural folds and groove. In the less advanced specimens the neural groove is open throughout its whole length, whereas by the end of the stage the groove is closed from the rhombencephalon to below the level of the last somites present.
The embryo is becoming longer, and the umbilical vesicle continues to expand. The rostral portion of the neural folds becomes elevated, a caudal fold begins to appear, and the whole embryo comes to rise beyond the level of the umbilical vesicle (i.e., a variable degree of lordosis usually becomes evident). By the end of the stage the cardiac region has become a prominent feature of the external form (Boyden, 1940).
Representative specimens of this stage are illustrated in figures 10-3 and 10-4. These outlines, showing the dorsal aspect of a median section of each specimen, are enlarged to the same scale. As pointed out by Bartelmez and Evans (1926), the mesencephalic flexure is present in all embryos of this period. A dorsal flexure may or may not be found. The curvature of the dorsal profile varies from a gentle convexity through all degrees of concavity (lordosis) from the least possible curve to a deep, sharp kink. Bartelmez and Evans (1926) and Streeter (1942) have discussed the significance of this variation as seen in human and rhesus embryos of early somitic stages. On the whole, the evidence indicates that, whereas extreme dorsal flexion should be regarded as an artifact, anything from a gentle convexity to a moderate dorsal concavity can be considered normal.
The optic sulcus develops in the forebrain and, toward the end of the stage, an indication of invagination of the otic disc is found. During stage 10, swellings begin to appear for the mandibular arch (Politzer, 1930, fig. 5), and the hyoid arch and probably the maxillary process become identifiable (Bartelmez and Evans, 1926, fig. 6). Pharyngeal cleft 1 becomes visible (Corner, 1929, figs. 4 and 10).The future ectodermal ring (O'Rahilly and Müller, 1985) is beginning to form as a thick area overlying pharyngeal arch 1. An indication of an intermediate band is present and represents the future intermembral part of the ectodermal ring.
Page 94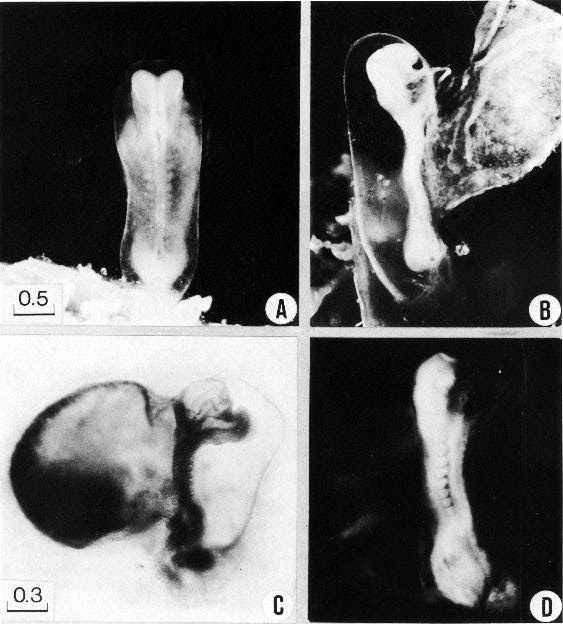
Fig. 10-1. Photographs of three embryos with 7–+12 pairs of somites. (A) In the dorsal view of No. 6330 the neural folds are well shown both in the brain and in the spinal area. The folds are actually fused in a short region of the embryo, as indicated in the outline sketches of the same specimen (figs, 10-3 and 4). The amnion and amniotic cavity are distinguishable. (B) The mandibular arch is visible in the lateral view of No. 6330. (C) The 8-somite embryo, H 98 (Wilson, 1914) or No. 7251 has an unusually abrupt upward bending of the rostral third of the body. The relations of heart, umbilical vesicle, and other details are evident. (D) The somites and the caudal eminence are well shown in No. 3710. Photographs A–C are enlarged to the same scale.
Page 95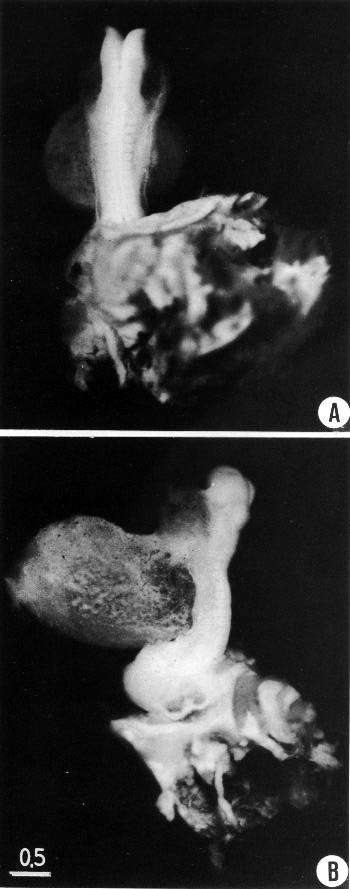
Fig. 10-2. Photographs of the 10-somite embryo No. 5074, made after most of the amnion had been removed. The general body form and structural details of the embryo are well shown in both the dorsal and lateral views. The neural closure extends a short distance caudal to the last somite, to the level of the otic segment ofthe hindbrain. The pericardial cavity is quite large in this specimen. In B the heart itself is faintly visible through the thin, translucent body wall.
Page 96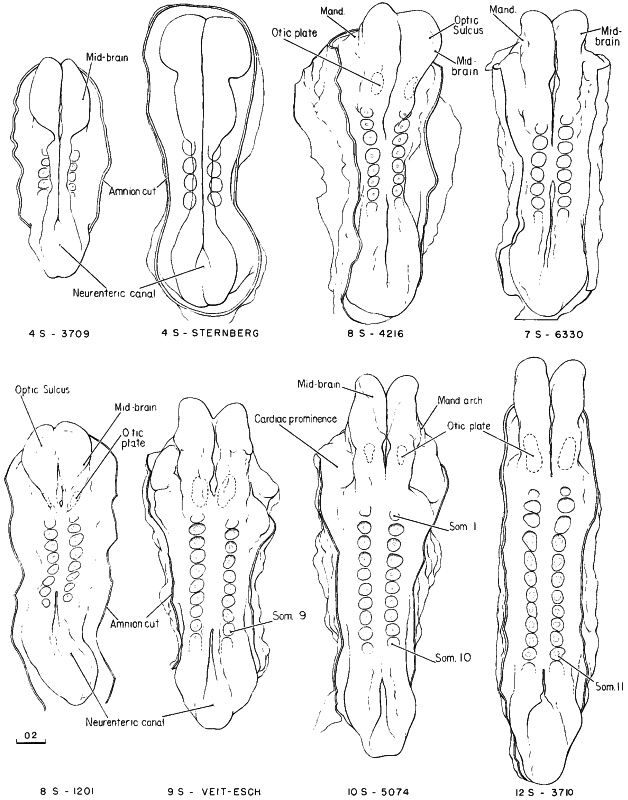
Fig. 10-3. Outline drawings showing, in dorsal view, eight embryos belonging to stage 10. The number of somitic pairs and the collection number are given for each specimen. The neural folds still gaping apart or separated from each other in the 4-somite specimens come together, and the fusion rapidly spreads rostrally into the region of the hindbrain and caudally about as far as the last somites formed; the rostral and caudal neuropores shrink, but they are still relatively large in the more advanced members of the group. The pericardial region, relatively small in the 4-somite embryos, is prominent in embryos of 30 or more somites. The tracing of one 4-somite embryo was made from figures by Sternberg. No. 6330 and Veit-Esch were drawn from the Born reconstructions. The others are drawings based on reconstructions and figures by Bartelmez and Evans (1926), Payne (1925), and Corner (1929).
Page 97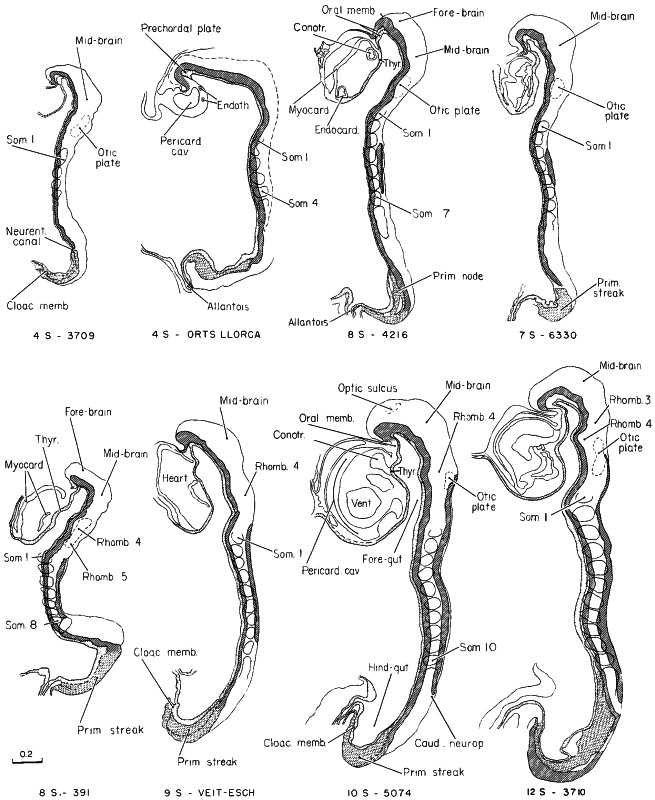
Fig. 10-4. Outline drawings of median sections of embryos belonging to stage 10. The head fold is prominent in all specimens shown, and the caudal fold is just beginning to appear in the 4-somite embryo; the hindgut shows the first sign of separating from the yolk sac. The line of fusion of the neural folds lengthens rapidly after 7 pairs of somites have appeared. In more-advanced embryos, the general form of the body is greatly influenced by the enlarging heart and pericardial cavity.
The drawing of one 4-somite embryo was slightly modified from a figure by Orts Llorca (1934). Data for the Veit-Esch embryo were obtained from a Born reconstruction and from a figure by Florian and Völker (1929). No. 6330 was drawn from a Born reconstruction. The others are drawings based on reconstructions and figures by Bartelmez and Evans (1926), Payne (1925), and Corner (1929).
Page 98HISTOLOGICAL FEATURES
Primitive streak. In stage 10 the primitive streak is limited to the caudal part of the body, an area that has been termed the caudal eminence (Müller and O'Rahilly, 1983) or future Endwulst (end bud). As illustrated in an informative scheme by Florian (1934b), the length of the primitive streak in relation to total embryonic length becomes smaller and smaller (Bartelmez and Evans, 1926; Müller and O'Rahilly, 1985). In stage 10 the primitive node “is about at the entrance of the hindgut” (Heuser and Corner, 1957).
Caudal to the neurenteric canal or its site (fig. 10-7a), dense axially located cells represent a part of the primitive streak. This area was formerly (Corner, 1929; Heuser and Corner, 1957) regarded as a portion of the notochord. The caudal end of the embryo consists of the hindgut and its thick endodermal lining, the primitive streak and an axial mesenchymal condensation adjacent to the site of the neurenteric canal, undifferentiated mesenchyme laterally, and, dorsally, an extension of the neural plate. A primitive groove is present only occasionally.
Somites. The number of somites increases during stages 10 and 11, and the rostralmost 4 are occipital. Somitocoeles are present but are no longer visible at stage 11. Sclerotomic cells are distinguishable at the ventromedial angle of the somite (Corner, 1929, fig. 25).
The average length of a somite is 80 µm (Müller and O'Rahilly, 1985). The number of presomitic spaces is 4–6.
It has been shown by Arey (1938, table 3) that the first somite is large (equals the second in size) in stages 9 and 10, up to 9 somites. From 10 somites to the end of stage 11, the first is generally much smaller than the second somite.
Notochordal plate. Although frequently referred to as the notochord, the axial cells caudal to the prechordal plate are still merely notochordal plate at this stage. The notochord sensu stricto is present only where notochordal cells have become completely separated from a continuous endodermal lining (Müller and O'Rahilly, 1985), and this does not occur until the next stage.
Rostral to the neurenteric canal, the notochordal plate is directly continuous with the endoderm and still forms a portion of the roof of the gut. In transverse section the plate begins to project dorsally, and U-shaped areas become increasingly extensive. The notochordal plate is in contact with the basement membrane of the neural plate or tube. Relatively few mitotic figures are evident, and they are mostly near the neurenteric canal or its site.
Neurenteric canal. Although a neurenteric canal soon ceases to be evident, at least its site can be recognized in all embryos of this stage (Müller and O'Rahilly, 1985, figs. 1–3).
Prechordal plate. The prechordal plate, which is constantly present, lies under cover of the rostral part of the prosencephalon, being separated by only the basement membrane of the brain. The plate is generally regarded as a source of proliferation of prechordal mesoderm.
The migration of prechordal cells began in stage 9, and prechordal mesoderm continues to form in stage 10. The rotation from a position in the longitudinal axis to a right angle had also already begun in stage 9 (Müller and O'Rahilly, 1983). Gilbert (1957) believed on morphological grounds that the prechordal plate is the source of those extrinsic muscles that are innervated by the oculomotor nerve. His data have been confirmed and completed by experimental work and by electron microscopy in the chick embryo. In birds the muscle cells of all extrinsic ocular muscles develop probably from the prechordal plate rather than from somitomeres (i.e., from paraxial and not axial mesenchyme). The relationship of the prechordal plate to the foregut may vary with species.
Umbilical vesicle. From stage 7 onward, the external and internal strata of the bilaminar umbilical vesicle are referred to as the mesodermal and endodermal layers, respectively. Three layers can be distinguished by electron microscopy at stage 10: mesothelium, mesenchyme, and endodermal epithelium (Hesseldahl and Larsen, 1969, 1971). The chorionic villi have also been investigated by electron microscopy (Knoth, 1968).
CARDIOVASCULAR SYSTEM
The pericardial cavity is always present and, in the more advanced specimens, a passage connects the intra- and extra-embryonic coeloms (Dandy, 1910. fig. 11; Corner, 1929. fig. 7). It is probable that the coelom Page 99 serves as a means for access of nutritive fluid to the embryonic tissues before blood vessels take over this function (Streeter, 1942).
In addition to such vessels as the umbilical arteries and veins, aortic arches 1 and 2 develop.
Heart
Cardiac contraction “is believed to commence at the beginning” of stage 10 (de Vries and Saunders, 1962) or at the end of stage 9.
The wall of the heart comprises a thin myocardial mantle, recticulum (“cardiac jelly”), and endocardium (Payne, 1925, fig. 10). A dorsal mesocardium is formed (Davis, 1927), and perforation of it may begin (Corner, 1929).
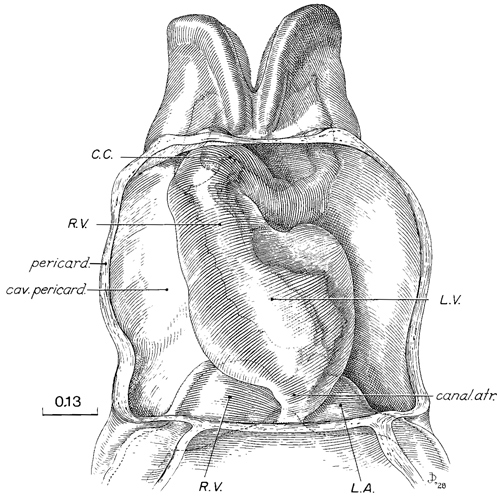
Fig. 10-5. The heart, ventral view, from a reconstruction in translucent material, showing pericardial cavity, myocardial mantle, and the endocardial tube. From Corner (1929). Labels modified according to current interpretation. C.C., conus cordis.
Three steps in the development of the heart can be recognized in stage 10 (fig. 10-6).
(l) The endocardial primordium is a plexus of delicate vessels lying on the foregut, and consists of two parallel channels interconnected by two or more small transverse vessels (Davis, 1927, fig. 12). The atria are widely separated from each other, and the more centrally placed cardiac elements (according to current interpretation) comprise, caudorostrally, the prospective left ventricle, prospective right ventricle, and conotruncus. The left interventricular sulcus is well marked on the surface of the heart (McBride, Moore, and Hutchins, 1981), so that the organ appears already to have lost its symmetry (de Vries, 1981).
(2) The two endocardial tubes become fused (Davis, 1927, fig. 22) so that a single tube now comprises caudorostrally, the left ventricle, right ventricle, and conotruncus. In addition, the cardiac loop forms when 7–20 pairs of somites are present. It is usually directed to the left and it includes ventral bowing and (when viewed from the front) counterclockwise rotation of Page 100 the ventricular segments (de Vries and Saunders, 1962). Although a sinus venosus as such is not present until the next stage, left and right sinusal horns appear during stage 10.
(3) The endocardial tube adopts a definite S-curve, the middle portion of which is formed by the ventricular loop, and hence the organ is now markedly asymmetrical (Davis, 1927, fig. 24).
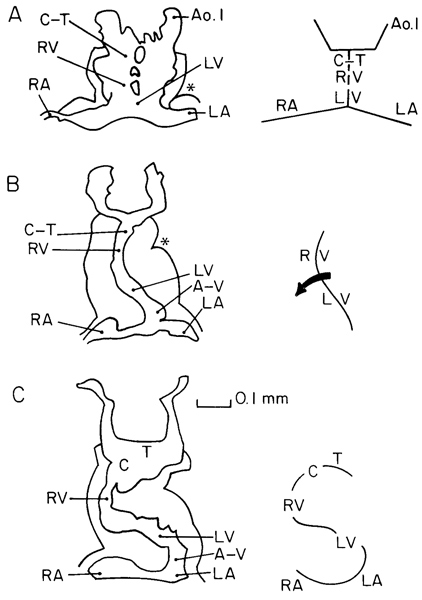
Fig. 10-6. Outlines of the myocardial mantle and endocardial tube showing three successive phases of cardiac development during stage 10. Schematic representations are shown on the right. A. plexiform phase (No. 3709). B, beginning cardiac loop (arrow)(No. 391). C. definite S-curve (no. 3707). The outlines are based on Davis (1927) with current (e.g., de Vries and Saunders, 1962) interpretation. Asterisk: left interventricular sulcus. A-V, atrioventricular junction. Ao.1, aortic arch 1. C, conus cordis. C-T, conotruncus. T, truncus arteriosus.
DIGESTIVE AND RESPIRATORY SYSTEMS
During stage 10, when mesenchymal cells are no longer found between the ectoderm and the endoderm at the summit of the foregut, an oropharyngeal membrane is present.
A small portion of the ectoderm adjacent to the summit (chiasmatic plate) of the neural plate is considered to be the primordium of the adenohypophysis.
Pharyngeal cleft 2 and pouch 2 are identifiable, and pharyngeal pouch 3 may be indicated. The thyroid primordium appears (O'Rahilly, 1983a). A median pharyngeal groove and ridge are present, and include the laryngotracheal sulcus. The pulmonary primordium appears at the caudal end of this sulcus, and the two constitute the respiratory primordium (O'Rahilly and Boyden, 1973). A transverse groove between the umbilical vesicle and the ventral surface of the pericardial cavity foreshadows the “cranial coelomic angle” (Gitlin, 1968). The hepatic plate has been identified as an endodermal thickening at the rostral intestinal portal, caudal and ventral to the heart (Severn, 1971, 1972). The caudal intestinal portal, and hence the midgut, is becoming delimited.
URINARY SYSTEM
The intermediate mesoderm becomes visible and, when 10 pairs of somites are present, the nephrogenic cord becomes differentiated from this mesoderm. However, “the concept of the pronephros does not apply to the human embryo” (Torrey, 1954).
NERVOUS SYSTEM
The rostral part of the neural plate is relatively flat at first but the neural folds soon become elevated, in association with the deepening neural groove. The elevation is related to (1) an increase in the amount of the underlying mesenchyme, caused by migration of mesencephalic crest and mitotic activity in situ, and (2) increasing size of the dorsal aortae and the aortic arches. The mesenchyme is believed to possess a supportive role during the initial phase of encephalic neurulation, and mesenchymal deficiency may prevent further neurulation.
Page 101The subdivisions of the rhombencephalon, which appear during stage 9, were clarified by Bartelmez (1923), whose system is followed here, with the addition of a more recently recognized segment D (Müller and O'Rahilly, 1983).
The terminal notch, which appears during stage 10 but is not always identifiable, indicates the telencephalic part of the forebrain (fig. 10-7b). This is the earliest stage at which the telencephalon has been identified. The telencephalic area represents the beginning of the lamina terminalis and therefore of the telencephalon medium (impar). The prosencephalic folds can be divided into an optic part (D1) and a postoptic part (D2). In the median plane, D1 is thicker than D2 and constitutes the primordium chiasmatis. In some embryos the optic sulcus continues medially and forms a slight indentation that indicates the future postoptic recess (primitive optic groove of Johnston, 1909). D1 shows many cellular inclusions. Parts D1 and D2 of the diencephalon are evident as “segments” (transverse partitions) up to stage 13 inclusive. In the median plane, D1 comprises the chiasmatic plate, D2 the area of the future neurohypophysis and the mamillary region. The mesencephalic flexure becomes reduced during stage 10, resulting in a ventral bending of the prosencephalon. The factors leading to flexure are believed to be intrinsic to the mesencephalon. The four divisions (A, B, C, D) of the rhombencephalic folds, first seen in the previous stage, are again distinguishable, and part D is the longest.
From stage 9 to stage 10 the prosencephalon increases in length, the mesencephalon remains the same, and the rhombencephalon decreases considerably. A correlation exists between the total length of the C.N.S. and the percentage of closed parts. Furthermore, rhombomere D and the spinal area grow relatively more, and these are the areas that close first. The spinal part of the neural plate increases fivefold in length. The elongation of the C.N.S. is related to the formation of new somites. Although this somite-related elongation is reflected chiefly in the spinal region, the cerebral portion lengthens also. The frequency of mitotic figures is highest in the spinal part of the neural plate and somewhat less in the rhombencephalon and prosencephalon.
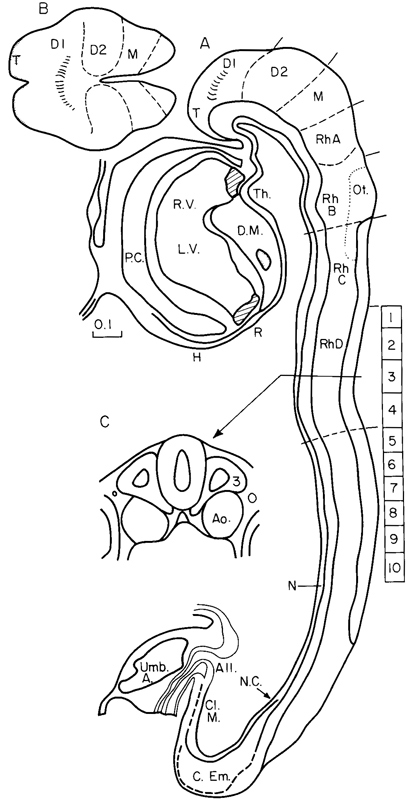
Fig. 10-7. (A) Median section of No. 5074 (10 pairs of somites, the levels of which are indicated by rectangles marked 1–10). Based partly on Corner (1929), with current (Müller and O'Rahilly, 1985) interpretation. (B) “Top” view of the rostral end of the embryo. The telencephalic portion is shaded and the optic sulci (in D1) are indicated. Based on Müller and O'Rahilly (1985). (C) Transverse section at the level of somites 3, showing neural tube, notochordal plate, dorsal aortae, and intermediate mesoderm.
Abbreviations. All., Allantoic diverticulum. Ao., Dorsal aorta. C.Em., Caudal eminence. Cl.M, Cloacal membrane. D1 and D2, Diencephalon. D.M. Dorsal mesocardium. H, Hepatic primordium. L.V. Left ventricle. M., Mesencephalon. N. Notochordal plate. Ot., Otic disc. N.C., Site of neurenteric canal. P.C., Pericardial cavity. R, Respiratory primordium. RbA–RhD, Rhombomeres. R.V., Right ventricle. T, Telencephalon medium. Th., Thyroid area. Umb.A. Umbilical artery.
Closure of the neural groove occurs first during stage 10, in embryos of approximately 5 paired somites. Page 102 The site of initial closure appears to be rhombencephalic (part D) or upper cervical, or both. It seems that closure may soon occur in several places independently, so that the process is not entirely comparable to a zip fastener. The maximum limits of closure found are to rhombencephalon 1 rostrally and to the level of somites 15/16 caudally. The caudal extension of the closure proceeds at about the same rate as the formation of new somites (Bartelmez and Evans, 1926), so that the margin of the caudal neuropore is usually opposite the latest pair of somites. Rostrally, progress is slower and apparently more variable.
The neural plate in stage 9 extends caudally to the level of the neurenteric canal. In stage 10 differentiation into neural plate extends beyond that landmark, as indicated by a radial arrangement of cells and separation from the underlying primitive streak by a basement membrane. The transition to the more caudally situated, undifferentiated ectoderm is gradual.
Cytoplasmic inclusions in the developing nervous system have been noted by several authors and have frequently been considered to indicate degeneration. Such inclusions are found in a number of areas, including the apices of the neural folds, the primitive streak, and the prechordal plate.
The histological features of neurulation have been investigated (Müller and O'Rahilly, 1985). In the spinal region, the neural folds approach each other and the surface ectodermal cells of the two sides make contact while a gap remains between the neuro-ectodermal cells of the two sides. Where fusion of the surface ectoderm and of the neuro-ectoderm has occurred across the median plane, a wedge-shaped area of neural crest is present in the dorsalmost part of the neural tube. Protoplasmic processes then protrude, and crest cells emerge from the tube. A more or less similar appearance of wedge-shaped neural crest and migrating cells is found also in part D of the hindbrain. Further rostrally in the hindbrain, and also in the midbrain and the forebrain, at the apices of the open neural folds, neural crest cells are emerging from the neurosomatic junction and also from the adjacent neural ectoderm in areas where the basement membrane is deficient. At the mesencephalic level, preparation for fusion is occurring. The surface ectoderm protrudes more medially, thereby overhanging the neuro-ectoderm. Certain intermediate areas that are still open have been misinterpreted as neuroschisis. The neural folds show a high alkaline phosphatase activity (Mori, 1959a). It is important to note that differences in the process of neurulation have been recorded in various species.
Neural Crest
The neural crest continues to develop during stage 10 when, in the head, it probably reaches its peak. The rostral (mesencephalic) and facial portions are constantly present. The crest material for the superior ganglion of the vagus appears before that for the glossopharyngeal. The probable succession of appearance is: facial, rostral (mesencephalie), trigeminal, vagal, occipital, and glossopharyngeal. The facial crest is the most conspicuous. In the area of the vagal crest, the neural crest material is clearly joined by cells of the surface epithelium in some of the embryos. The otic plate, at least in some instances, may be seen to contribute cells to the facial (acousticofacial) crest. The cells of the neural crest appear to be derived from the open neural plate at the neurosomatic junction, mostly in areas where the neural tube has not yet formed. Apart from its formation of cranial ganglia, the neural crest migrates into the head mesenchyme and is believed to contribute to the skull and face. Illustrations purporting to show precisely the extent of the contributions in the human (based on the chick embryo) are quite unwarranted in the present state of knowledge.(1) The proposal of failure of crest cell formation as a facial pathogenetic mechanism has been disputed. Neural crest cells clearly leave the neural plate at areas where the basement membrane is interrupted. After closure of the neural groove in stage 10, neural crest still seems to be derived from the neural ectoderm, although a simultaneous origin from the surface ectoderm cannot be excluded.
(1) It has frequently been pointed out that the “static” appearances seen in serial sections cannot justifiably be used for the interpretation of “dynamic” processes. It has less frequently been emphasized that there are equally grave problems in assuming that the results of experimental embryology can be transferred without more ado to the human embryo.
Page 103Eye
By approximately 7–8 paired somites the neural folds of D1 consist of thick neural ectoderm that comprises the optic primordium. Identification of the optic primordium is difficult, and reconstructions are necessary. The thickened area, which contains many mitotic figures and cytoplasmic inclusions, continues across the median plane as the chiasmatic plate (primordium chiasmatis). A more or less indented area in D1 represents the optic sulcus (fig. 10-7). In contrast to the shallow ventricular surface in D1, the ventricular surface in D2 is convex and represents the future thalamic area of the diencephalon. The optic sulcus does not always reach the median plane, but, when it does so, it indicates the future postoptic recess (Johnston, 1909). The area rostral to the optic primordium on both sides of the terminal notch represents the telencephalic primordium (fig. 10-7B). Some of the earlier specimens in which an optic primordium or sulcus has been described are unfortunately not of sufficient histological quality to avoid the suspicion that artifacts are present. Moreover, the plane of section is of the utmost importance in the identification of the optic region. D2 gives rise later to the thalami, but it is not correct to state that “the entire dorsal thalamic wall... had originally been incorporated in the optic evaginations” (Bartelmez and Blount, 1954). Careful plotting of the optic sulcus in the Payne embryo shows that it is confined to D1 and is transverse in direction, in contrast to the vertical markings shown by Payne (1925, fig. 2). Similarly in the Corner embryo, the optic sulci (which do not quite meet in the median plane) are limited to D1, and the optic primordia (i.e., the surrounding thickenings) occupy more or less the whole of D1. The lateral limit of each optic sulcus appears to be beginning to extend caudally. Corner (1929, fig, 1) plotted the sulcus more caudally, probably because of the influence of the early interpretations of Bartelmez (1922). An improved plotting of the Corner embryo was illustrated by Bartelmez and Dekaban (1962, fig. 67), although the midbrain was placed too far rostrally, thereby not allowing adequately for D2. By the end of stage 10, the optic sulci extend more caudally, as in the Litzenberg embryo (Boyden, 1940, fig. 13), in which the label superior colliculus should read thalamic primordium in D2.
Ear
The otic plate in at least one embryo contributes cells to the facial (or faciovestibular) neural crest. Such a contribution continues to at least stage 12 (O'Rahilly, 1963). It may be that the non-neuronal cells in the vestibular and cochlear ganglia are derived from the neural crest.
SPECIMENS OF STAGE 10 ALREADY DESCRIBED
4 somites, Carnegie No. 3709 (University of Chicago H 279). Characterized with outline sketches, by Bartelmez and Evans (1926).
4 somites, Histologisch-Embryologisches Institut, Embryo A, Vienna. Fully described by Sternberg (1927).
4 somites, Histologisch-Embryologisches Institut, Embryo Ca, Vienna. Fully described by Orts Llorca (1934).
4–5 somites, Florian's Embryo Bi II. The whereabouts of this, the following embryo, and the 10-somite Bi XI (see below) are not known; Florian's collection has not been found since his untimely death during World War II. The embryo Bi II was briefly characterized by Studnicka (1929), cited and partly illustrated by Florian (1928, 1930a).
4–5 somites, Florian's Embryo Bi III. (See note on previous embryo.) Briefly characterized by Studnicka (1929) and cited by Florian (1928).
4–5 somites, Carnegie No. 2795. Cited and briefly characterized by Bartelmez and Evans (1926). The specimen is distorted and somewhat macerated.
5 somites, Anatomisches Institut, Zürich, GM 1954. Described and illustrated by Schenck (1954).
5 somites, No. 103, Department of Anatomy, Tohoku University, Sendai. Distribution of alkaline phosphatase studied by Mori (1959a) in this and in another (No. 101), possibly 8-somite, embryo.
5–6 somites, Pfannenstiel “Klb” (originally at Giessen; was in Keibel's Institute at Freiburg i. Br. about 1911, may now be in Berlin). This well known embryo is No. 3 in the Keibel and Elze Normentafel (1908). Models by Kroemer (1903). A partial set of tracings made by H. M. Evans is in the Carnegie Collection, No. 5463.
6 somites, Carnegie No. 8244. Somewhat distorted; histologically fair.
6 somites, University of Michigan No. 71, Ann Arbor. Briefly described by Arey and Henderson (1943). A full description in an unpublished doctoral dissertation is in the files of L. B. Arey at Northwestern University, Chicago.
6 somites, Carnegie No 8818 (University of Chicago H 338) Pathological, not used in present study. Listed here because cited by Bartelmez and Evans (1926).
6–7 somites. His’s Embryo “SR.” Cited by His (1880) and Page 104 by Bartelmez and Evans (1926). Has been studied only in the gross.
6–7 somites, Embryo LM (present location unknown). Cited here from manuscript notes at Carnegie laboratory, made from Russian text of Burow (1928). Condition said to be poor.
7 (?) somites, Embryo “Ludwig,” Berlin. Described by Streiter (1951). This specimen, which is somewhat macerated, is in certain characteristics considerably in advance of others of similar somitic number.
7 somites, Carnegie No. 6330 (University of Chicago H 1404). Extensive manuscript notes on this specimen, made under the supervision of G. W. Bartelmez, are in the files of the Carnegie laboratory.
8 somites, Carnegie No. 4216. Described by Payne (1925), and very frequently cited.
8 somites, Dublin. Described by West (1930); see also Arey (1938). Photographs and models are in the Carnegie Collection, No. 4923. Bartelmez (personal communication) thinks that this distorted embryo had only 5–6 somites.
8 somites, Carnegie No. 391. Described by Dandy (1910) and frequently cited (cf. Bartelmez and Evans, 1926, with additional illustrations). There were neither camera drawings nor photographs of the intact specimen, and therefore the reconstructions are not entirely satisfactory. The plaster models now at the Carnegie laboratory were made by O. O. Heard under the supervision of Bartelmez for the paper by Bartelmez and Evans (1926). The apparent lack of fusion of the neural folds described by Dandy is an artifact produced by a crack.
8 somites, Carnegie No. 1201 (University of Chicago H 87). Described briefly by Evans and Bartelmez (1917); cited, with illustrations, by Bartelmez and Evans (1926).
8 somites, Embryologisches Institut, Embryo Ct, Vienna. Fully described by Politzer (1930). Arey (1938) counts 8 paired somites in this embryo instead of 7 as stated by Politzer.
8 somites, University of Cambridge, Department of Anatomy H 98. Photographs and models in Carnegie Collection, No. 7251. Described by J. T. Wilson (1914). Cited by Bartelmez and Evans (1926), who consider it slightly abnormal in form although good histologically.
9 somites, Embryo “Esch I,” Marburg. Elaborately described by Veit and Esch (1922), and cited, with illustrations, by Bartelmez and Evans (1926), who count 9 somites instead of 8 as stated by the original authors. Chorionic villi studied in detail by Ortmann (1938). Photographs and models are in the Carnegie Collection, No. 4251.
9 somites, Embryo “Du Ga,” Geneva. Described by Eternod (1896); models by Ziegler were distributed commercially. Cited by Bartelmez (1922) and Bartelmez and Evans (1926), with illustrations. Tracings made by H. M. Evans at Geneva and models are in the Carnegie Collection, No. 4439.
About 9 somites. Embryo Unger, Keibel Collection, Freiburg i. Br., No. 4 of Keibel and Elze (1908). Listed by Bartelmez and Evans (1926).
9 somites, Embryo “Jacobsen,” formerly at Kiel (Graf Spee's collection was destroyed in World War II). Described by von Spee (1887). Listed by Bartelmez and Evans (1926) as having “at least” 9 somites.
9 somites, Embryo Ca of Orts Llorca, Madrid. Various details described by Mari Martinez (1950) and Martinez Rovira (1953).
9–10 somites, Embryo R. Meyer 335. (Robert Meyer's collection was purchased by the late Hedwig Frey and bequeathed by him to the Anatomisches Institut, University of Zurich.) Listed by Bartelmez and Evans (1926), and cited by Felix (1912).
10 somites, Da2, Anatomical Institute, Basel. Described by Ludwig (1929). Plastic reconstructions. Neural groove closure extends rostral to otic discs.
10 somites, Carnegie No. 5074 (University of Rochester H 10). Fully described by Corner (1929), and subjected to volumetric analysis by Boyden (1940). Excellent specimen.
10 somites, Grosser's Embryo Schwz (present location unknown). Briefly described, without illustrations, by Treutler (1931). Preservation said to be not altogether satisfactory.
10 somites, Florian’s Embryo Bi XI. (See note on Bi II above.) Briefly described, with illustrations, by Politzer and Sternberg (1930); cited and partly illustrated by Florian (1930a).
10 somites, Anatomy Department, University of South Wales, Cardiff. Partly described and illustrated by Baxter and Boyd (1939).
11 somites, Embryo T 152, University of Toronto, Department of Anatomy. Cited by Arey (1938).
11 somites, Embryo G-dt, Uppsala. Described by Holmdahl (1943) as having 11 well-differentiated pairs of somites, with beginning delimitation of 4 more.
11–12 somites, Carnegie No. 8970 (University of Chicago H 637). Somewhat damaged. Cited, with illustrations, by Bartelmez (1922) and Bartelmez and Evans (1926).
12 somites, Carnegie No. 3710 (University of Chicago H 392). Cited by Bartelmez (1922) and Bartelmez and Evans (1926).
12 somites, Carnegie No. 3707 (University of California H 197). “Legge embryo.” Cited, with illustrations, by Bartelmez and Evans (1926). The coital history accompanying this specimen, which was declared to be reliable, would give it a postovulatory age of either 18 or 39 days; the former seems rather brief but the latter is much too long.
12 somites, Litzenberg embryo, University of Minnesota, Minneapolis. Briefly described by J. C. Litzenberg (1933); characterized and subjected to volumetric analysis by Boyden (1940), who counts 12 somites instead of 13–14 as in the original description. Photographs and model in Carnegie Collection, No. 6740.
12 somites, M. 24, University of Michigan, Ann Arbor. Cited by Arey (1938).
Copyright © 1987 Carnegie Institution of Washington. Reproduced on ehd.org with permission.
