Developmental Stages in Human Embryos
Go to Stage: Intro 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Stage 18
Page 220Approximately 13–17 mm
Approximately 44 postovulatory days
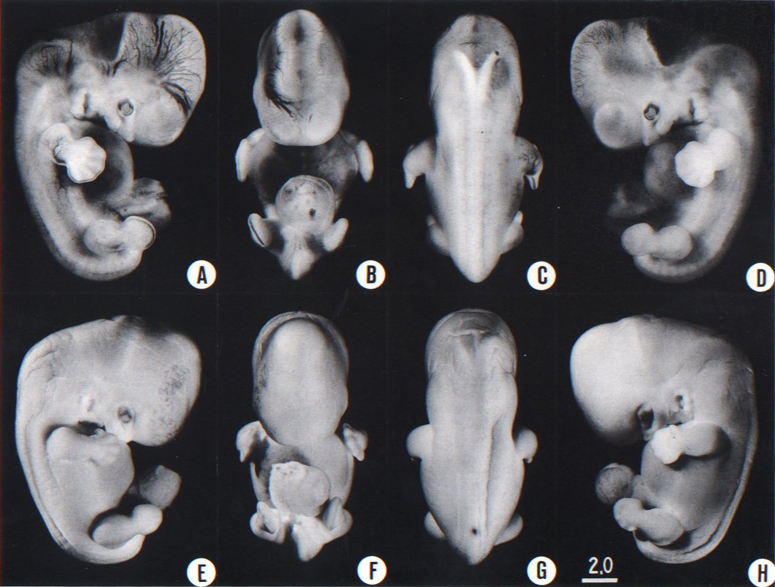
Fig. 18-1. (A-D) Four views of a less advanced member (No. 8097) of stage 18. One can see the vascular patterns and details of inner structure. The inner ear, and also the trigeminal nerve and details of the eye, can be seen in the profile views. A number of details in the form of the brain, notably the cerebral hemispheres, are discernible (cf O'Rahilly, Müller, and Bossy, 1982)
(E-H) Four views of a more advanced member (No 7707) of stage 18. Because of the particular fixation, the tissues are opaque, making the specimen suitable for the study of surface features. The embryo has become wider from side to side, and it is now more or less cuboidal (F,G). The finger rays have become more distinct, and the rim of the hand plate is moderately crenated; one can recognize the bend of the elbow. Toe rays are visible (H). Small condensed masses are creeping forward over the retina, and the upper eyelid is beginning. The three-hillock plate, to the rear of the hyomandibular groove, exhibits different stages in its resolution into definitive parts of the external ear.
Page 221SUMMARY
External: the body is more of a unified cuboidal mass, and both cervical and lumbar flexures are indicated; the limbs are longer, the digital plate of the hand is definitely notched, the elbow region is usually discernible, and toe rays can be identified in some specimens; eyelid folds are present in the more advanced embryos; a distinct tip of the nose can be seen in profile; auricular hillocks are being transformed into specific parts of the external ear.
Internal: in the heart, septum secundum and the associated foramen ovale are appearing; the membranous part of the interventricular septum is beginning to take form; the vomeronasal organ is represented by a groove; choanae develop; some subsegmental buds develop in the bronchial tree; collecting tubules develop from the calices; testicular cords may begin to appear in the male gonad; the paramesonephric duct grows rapidly down through the mesonephros; 1–3 semicircular ducts are present in the internal ear.
SIZE AND AGE
Less-advanced embryos of stage 18 may be expected to have a greatest length of about 14.5 mm, whereas more-advanced examples would in most instances be about 16 mm. Nearly two-thirds of all embryos at this stage range from 14 to 16 mm.
Although the data are not extensive (only eight specimens), the greatest diameter of the chorion usually ranges from 40 to 51 mm, and all three principal diameters are approximately equal.
The age is believed to be approximately 44 postovulatory days.
EXTERNAL FORM
From external form alone it is not always possible to distinguish between less-advanced specimens of stage 18 and more-advanced ones of stage 17. In drawing an arbitrary borderline between the two groups, as has been done in this study, one must rely on selected structural characters of the internal organs that are revealed in sections. Indeed, in several instances, it has been found necessary to change the placement of certain embryos from a provisional one based on their external form to one in an adjacent group, based on the study of the same embryos after they had been cut in serial sections.
To gauge accurately the level of development of an embryo, its internal structure must be taken into account. There are many things, however, to be learned from the external form. One can see, for instance, that the group of embryos shown in figure 18-2 is more advanced than the similar group of the preceding stage, shown in figure 17-2. The embryo of stage 18 is larger and has advanced in the coalescence of its body regions, which have now become more closely integrated in a common cuboidal bulk, no longer consisting of individual and obviously separable parts.
Page 222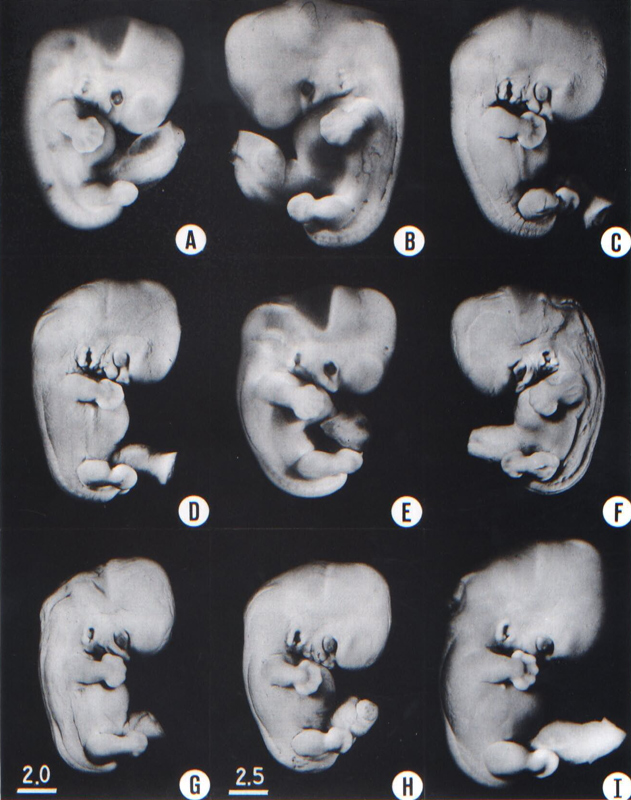
Fig. 18-2. Photographs illustrating the variations in form encountered in stage 18. It should be appreciated that certain appearances are the result of fixation, shrinkage, and the original condition of the embryo. All these specimens have been sectioned and in their microscopic structure all qualify as members of this stage, in which semicircular ducts are acquired by the inner ear, in which the paramesonephric duct descends along the margin of the mesonephros, and in which the gonads show initial sexual differentiation. In general the embryos in the upper part of the plate are less advanced and those toward the lower part are more advanced. The embryo shown in I is near the borderline. It was fixed in a corrosive acetic mixture,which accentuates its surface features. The right nipple is visible immediately below the forearm. (A) No. 1909. (B) No. 5935A. (C) No. 6525. (D) No. 6528. (E) No. 5542B. (F) No. 6533. (G) No. 6522. (H) No. 6529. (I) No. 4430. B, C, D, F, G, and I are at the same magnification, whereas A, E, and H are slightly less magnified.
Page 223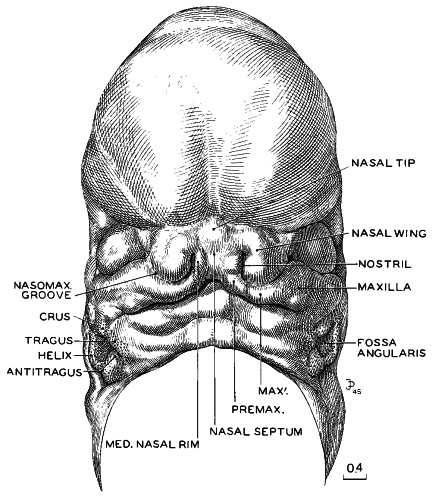
Fig. 18-3. Three-dimensional reconstruction of the face of No. 492, a typical representative of stage 18. By this time the nose and future upper jaw are definitely outlined. There is still a small gap between the right and left upper jaws, which will be smoothed out by the further enlargement and blending of the two premaxillary centers. When that occurs, the nasal septum will be closed off from the oral opening. It is still too early to speak of lips. The growth center marked MAX' is a subdivision of the maxillary center and is distinct from the lateral nasal process. Between the two is the nasomaxillary groove, which is commonly said to indicate the origin of the nasolacrimal duct, but Politzer (1936) disagreed. This figure forms a series with figures 15-3, 16-5, and 17-3. They are all enlarged to the same scale and hence are directly comparable. Drawing made by James F. Didusch. Reconstruction made by Osborne O. Heard.
The hands in all specimens have distinct finger rays and interdigital notches on the rims. In the more advanced half of the group, one can begin to speak of an elbow. The feet in some specimens exhibit toe rays, but the rim is not definitely notched. Even in some of the more advanced members one can still find a trace of somitic elevations in the sacral region.
All the embryos illustrated in figures 18-1 and 18-2 have been serially sectioned and studied histologically, and all of them conform microscopically to the criteria arbitrarily set for stage 18. The two embryos in figure 18-1 are typical specimens taken respectively from among less-advanced and more-advanced members of the group. The embryos in figure 18-2 illustrate such variations as are incident to differences in photography, methods of fixation, and variations in the degree of development encountered within this stage. The most advanced specimen (fig. 18-2,I) is on the borderline of the next stage.
Face
Above and below the eye one sees in more-advanced specimens of this stage the early rudiments of the eyelids (more marked in stage 19: Pearson, 1980) and the grooves initiating the conjunctival sacs. The thin outer lamina of the retina now contains considerable pigment, causing the retinal rim to be conspicuous. This is particularly true in formalin-preserved specimens, where the tissues are translucent. In embryos of stage 18 this pigmented rim of the optic cup is characteristically Page 224 polygonal, partly because of the forward projection of the upper margin of the retina and partly because of the retinal fissure. At the same time, white opaque zones of mesenchymal condensation spread progressively forward over the pigmented area, especially above and lateral to it. These opaque thickenings mark the laying down of the sclera and its muscular attachments.
In profile views of the nasal region one can begin to recognize the tip of the nose and the frontonasal angle, from which point the future bridge of the nose is to extend “forward.” To see the face satisfactorily one must study either decapitated specimens or reconstructions of the facial region made from serial sections. Such a model of a representative embryo of stage 18 is illustrated in figure 18-3. The nose, nostrils, nasal tip or apex, nasal wings, and nasal septum (columella nasi) can be identified clearly. The nose at this time can be thought of as a raised window awning; later, as the bridge of the nose forms, the awning will be let down. What is now in a vertical plane will then be horizontal. The upper lip is not differentiated as a separate structure. It will be formed jointly and in segments by the premaxillary and maxillary centers of the two sides. In this same figure the auricular hillocks are visible, although they are seen better in profile views (figs. 18-1 and 18-2). These hillocks were prominent elevations in the preceding stage. Here they are merging with one another and the adjacent surfaces, thereby forming the primordia of definite parts of the auricle. Those rostral to the auditory cleft are more advanced than those caudal to it. Thus the two dorsal hillocks (Nos. 2 and 3) of the mandibular arch are losing their individuality in the process of fusing to form the crus helicis. In more-advanced specimens of the group, the two dorsal hillocks (Nos. 4 and 5) caudal to the cleft have similarly merged to become the helix. Hillocks 1 and 6 persist and become, respectively, the tragus and antitragus, with the lobule pendent from the latter.
Nasal Passages
The nostrils (fig. 18-4) are the rims of the right and left nasal depressions, that is, lines of junction of nasal disc and skin ectoderm. The depressed center of each disc takes the form of a deep pocket, and, as a result of the growth of its own epithelium and the enveloping effect of the proliferating surrounding tissues, it becomes a supplementary air passage. This passage at first ends blindly, but in the more advanced members of this stage its blind end opens through what had been the temporary bucconasal membrane and participates in the formation of the funnel-like transition into the pharynx. A communication between the respiratory system and the surface of the face is in this way provided. It bypasses the mouth, leaving the latter free to perform its own special functions.
Transverse serial sections through the nasal passages of two embryos belonging to this stage, one slightly less advanced than the other, are shown in figure 18-4. In the first one (No. 6524) it can already be seen that the walls of the passage are differentiated into regions, and that the ethmoidal epithelium (upper part of medial wall) is becoming distinct from the maxillary territory of the lateral wall. The shallow fold in the lower part of the medial wall marks the vomeronasal organ (fig. 18-14). These regional demarcations are still more evident in the second embryo (No. 4430). The epithelium, like that of the gut, is characterized by mosaic-like organ-forming regions, the boundaries of which are evident very early.
In these serial sections one can trace the fate of the nasal fin. In stage 17 it appeared as a plate of epithelium that maintains a connection between the nasal disc and the ordinary surface epithelium, in line with the groove between the maxillary and premaxillary growth centers. In transverse sections the nasal fin has the appearance of a ventral stem for the nasal passage. In the present stage, in the more rostral sections, it becomes disconnected and is taken up by the overlying nasal epithelium. Caudally, this fin cleaves open to form part of the choanae. In doing so it becomes stretched to form the bucconasal membrane, and this in turn is detached and absorbed in the wall of the choana, leaving a free respiratory passage. The topographical relations of the nasal passage are further clarified by sagittal sections.
Two embryos thus sectioned, a less advanced (No. 6525) and a more advanced one (No. 144), are shown in figure 18-4. Although in the more advanced of these two the respiratory bypass is an open channel, its mature relations are not yet established. These will be attained through differential growth which is accompanied by a relative shifting forward of the tongue and lower jaw.
Page 225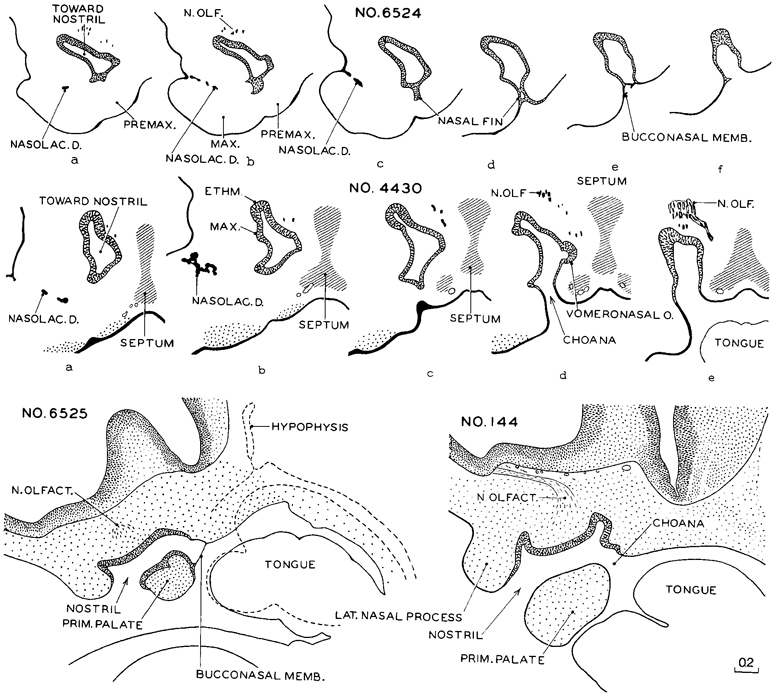
Fig. 18-4. Nasal passages in embryos of stage 18. The upper row shows transverse sections, at equal intervals, through the right nasal passage of a less advanced member of the group (No. 6524). Section a is the most rostral and f is the most caudal. In the caudal levels (d–f) the nasal epithelium, by means of its fin-like growth, has retained its continuity with the surface (oral) epithelium. Instead of remaining a solid plate, the nasal fin is now splitting to form the choana, its last remnants producing the bucconasal membrane.
The middle row (No. 4430) represents a more advanced member of the group, and the nasal epithelium shows specialized regions. The ethmoidal field is distinct from the maxillary field, and the choanal field differs from both of these. A definite vomeronasal organ is present (section d). The nasomaxillary groove is commonly said to indicate the origin of the nasolacrimal duct, but Politzer (1936) disagreed. A mesenchymal condensation now marks the primordium of the median cartilaginous septum of the nose.
The two sagittal sections of the lowest row show the final step in the establishment of a through nasal passage (the respiratory bypass). On the drawing of No. 6525 is superimposed the outline of a median section, indicated by broken lines, showing the relative position of the tongue. The drawing of No. 144, a more advanced embryo, is a composite of two consecutive sections. All drawings are at the same scale.
Page 226The initiation of the nasolacrimal duct is shown in figure 18-4 (No. 6524 b and c, and No. 4430 a and b). An irregular lamina or strand of epithelium is seen sprouting from the under-surface of the nasomaxillary groove. This epithelial strand descends through the mesenchyme, and later joins the lateral wall of the inferior meatus of the nose. The transformation of this strand into a duct and the junction of its upper end by two small canals with the conjunctival sac occurs later in prenatal life. Politzer (1936) disagreed with the usual account of the early development of the nasolacrimal duct.
CARDIOVASCULAR SYSTEM
Heart
An important feature is the appearance of septum secundum and hence the beginning of the foramen ovale at stages 18–21.
The heart is now a composite, four-chambered organ with separation of the pulmonary and aortic blood streams. In the more advanced members of the group the secondary interventricular foramen may already have closed, producing the future membranous part of the interventricular septum. The complete closure of the secondary interventricular foramen takes place during stages 18–21 (i.e., separating the cavity of the left ventricle from that of the right). The final boundaries of the foramen are the conal septum (particularly the right conal ridge) and the fused atrioventricular cushions (Wenink, 1971).
The aortic and pulmonary valves have acquired increased definition and are becoming cup-shaped. The tricuspid and mitral valves are later in their differentiation, but one can now recognize the ridges of condensed mesenchyme that are to form them. It is probable that they already have some valvular function.
Drawings of selected sagittal sections of a typical heart from one of the more advanced members of the group are shown in figure 18-5. In the condensing mesenchyme of the primary cardiac tube one sees, in such sections, sheets or areas of more-condensed tissue, shown in the figure by hatched lines, which underlie the endothelium and mark the proximity of cleavage and of adjustment of the walls.
From a coronal series of sections of another embryo at a similar level of development, a transparent reconstruction was made of the heart. Five study slabs (A–E) are illustrated in figure 18-6. The location of the front surface of each slab is indicated on the profile view of the heart, shown in the lower right corner. In this figure, as in the preceding one, the ventricular musculature is shown in solid black to distinguish it as a specialized tissue from the condensed mesenchyme of the primary cardiac tube and the venous type of muscular wall of the atria. In slab A one sees the nature of the future membranous part of the interventricular septum separating the right and left ventricles. In slab B, directly behind it, is shown the fusion of the wall of the primary cardiac tube, which thereby separates the tricuspid canal from the aortic corner of the left ventricle. In slab C is shown the complete separation of the aorta and pulmonary trunk, as far as the endothelium is concerned. Adjustments, however, are still in progress in the muscular and connective-tissue coats. The tricuspid and mitral passages are indicated. In slab D the sinus venosus is shown as it opens into the right atrium, immediately to the rear of the inferior vena cava. The two freely open at the same time, through the foramen ovale into the left atrium. In slab E is shown the manner of entrance of the superior vena cava. It is thus clear that from these three sources (sinus venosus, inferior vena cava, and superior vena cava) practically the whole of the incoming blood now arrives at the entrance of the foramen ovale.
Reconstructions of the heart at stage 18 have been illustrated by Kramer (1942, fig. 9) and by Vernall (1962).
DIGESTIVE SYSTEM
The esophagus shows distinct muscular and submucous coats. The fundus of the stomach begins to develop at about stages 18 and 19.
RESPIRATORY SYSTEM
The vomeronasal organ is represented by a groove in the medial wall of the nasal cavity (fig. 18-14). Choanae are being produced by the breakdown of the bucconasal membrane. The larynx, which became definable Page 227 in the previous stage, is now undergoing specialization (O'Rahilly and Tucker, 1973).
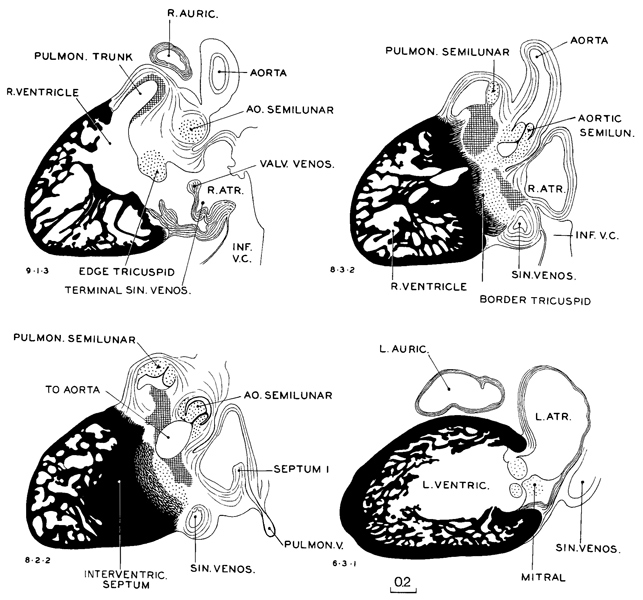
Fig. 18-5. Sagittal sections through a heart (No. 406) typical of stage 18. The interventricular septum is complete. The semilunar valves are now distinctly indicated. The tricuspid and mitral valves are scarcely more than rounded ridges of gelatinous reticular tissue. The latter tissue is last seen in these valves.
The trachea possesses a dense connective-tissue coat (fig. 15-7D) and is markedly different from the esophagus in both its epithelium and its supporting wall.
The pattern of the bronchial tree is shown in figure 18-14. The segmental bronchi are well defined (Wells and Boyden, 1954, plate 2), and a few subsegmental buds appear. In the embryonic period proper the left lung is said to lag behind the right lung in size and degree of development.
Page 228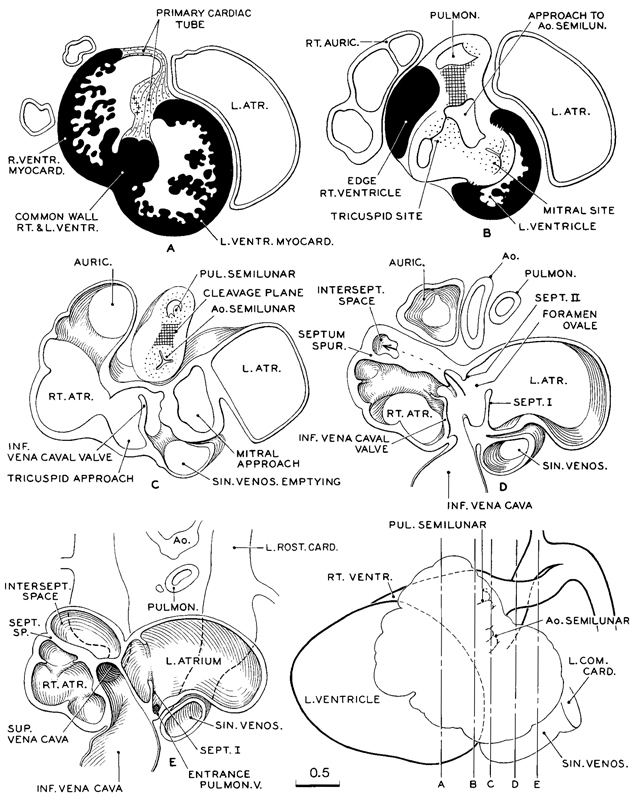
Fig. 18-6. Transparent reconstruction of a heart (No. 492) typical of stage 18. In the lower right-hand corner the heart is shown in left profile. The vertical lines through it indicate the front surface planes of the live transparent slabs shown separately as A–E. Each slab consists of a series of cellulose acetate sheets, properly oriented and spaced to give the correct third dimension. Tentative assembly showed that for reasons of clarity the slabs should not be thicker than is shown in the adopted levels. It will be noted that, although the separation of aortic and pulmonary channels has been effected, the peri-endothelial coats are still more or less blended, from the semilunar valves proximally. Peripherally they are separate and have acquired their individual walls, no trace is left of the gelatinous reticulum. Ventricular myocardium is shown in solid black. Cleavage planes are shown by crossed lines.
URINARY SYSTEM
Collecting tubules develop from the calices at stages 17 and 18 (fig. 18-14). They are surrounded by sharply outlined condensed primordia in the process of forming secretory tubules. Renal corpuscules are not yet present. By stage 18 the mesonephric duct and the ureter open almost independently into the vesico-urethral canal (Shikinami, 1926): i.e., the common excretory duct is disappearing. The cloacal membrane is ready to rupture.
Page 229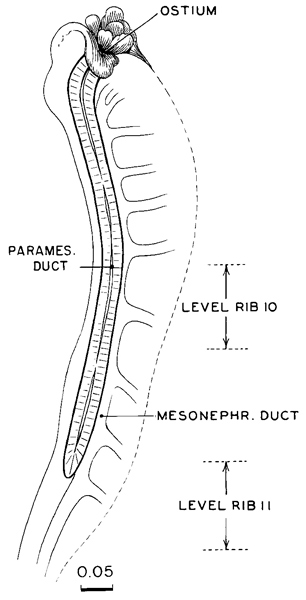
Fig. 18-7. Topography of the paramesonephric duct in a more advanced specimen (No. 406) of stage 18. The rib level refers to its free end and can be determined only approximately. Drawing made by James F. Didusch.
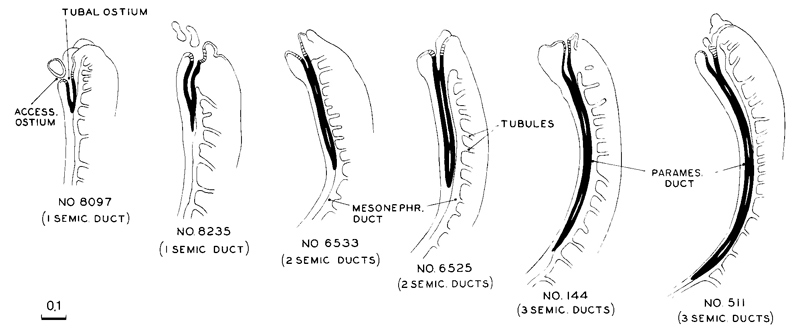
Fig. 18-8. The caudal extension of the paramesonephric duct incurring during stage 18 less advanced specimens are toward the left and more advanced ones toward the right. Their order is based on the length of the duct, and this is found to correspond closely to other developmental characters. For example, in the less advanced ones only one semicircular duct is established in the inner ear, whereas the more advanced ones have the full complement of three semicircular ducts
Page 230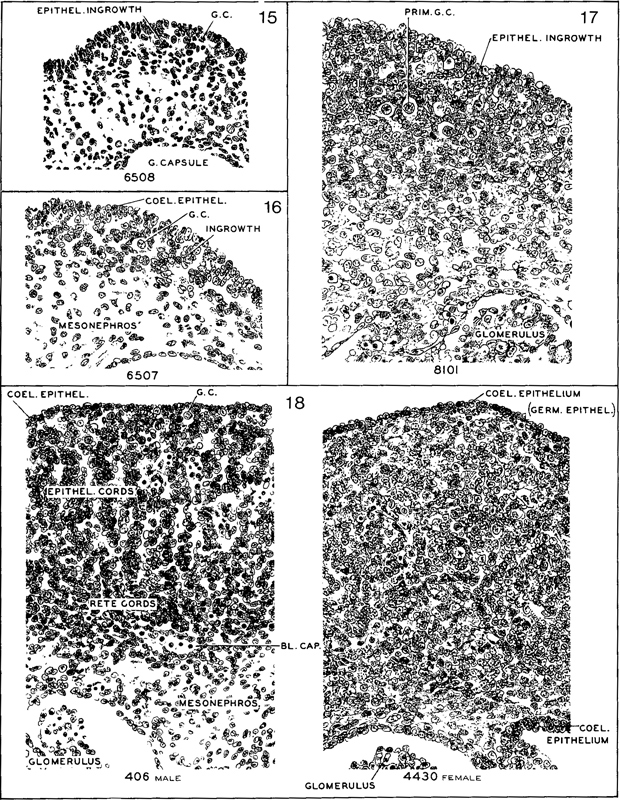
Fig. 18-9. Comparable sections passing transversely through the gonad in embryos selected astypical of stages 15–18. The first three stages show progressive phases of invasion and proliferation of the coelomic epithelium, providing the gonadal framework and follicular clusters around the primordial germ cells. Corresponding to the invasion, the surface is irregular, and one does not find a discrete surface layer of “germinal epithelium” before stage 18. Among the more advanced members of stage 18, one recognizes initial sexual differentiation. An example is shown of the male type of epithelial cords with active angiogenesis (No. 406) and of the female type (No. 4430). In both of these the surface of the gonad is smooth, and there is a distinctly marked-off layer of “germinal epithelium.” Outlines were traced on photographic prints which were subsequently bleached. Section numbers are as follows: No. 6508, 35-2-5; No. 6507, 11-1-4; No. 8101, 29-4-6; No. 406, 41-1-4; No. 4430, 24-4-6. All are at the same magnification.
Page 231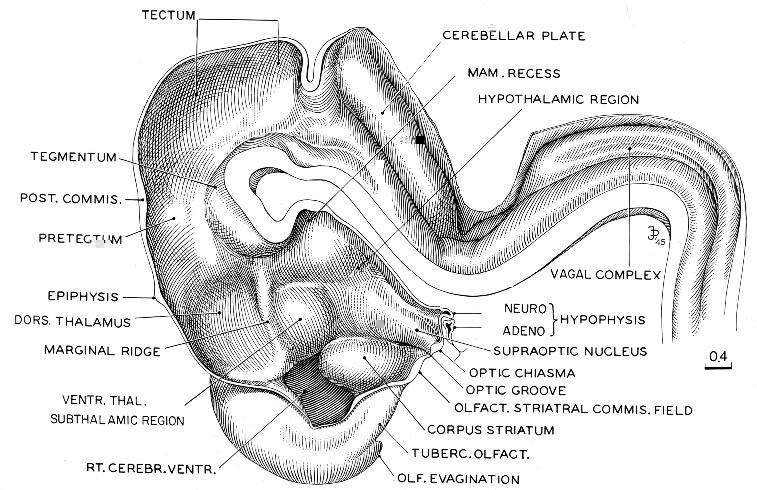
Fig. 18-10. Reconstruction of right half of brain of embryo (No. 492) belonging among more-advanced members of stage 18. The basal plate continuing from the spinal cord and extending into the tegmentum has become thicker, and in transverse section the hypoglossal region begins to resemble sections of the mature hindbrain. The dorsal parts of midbrain and diencephalon are still backward compared with the basal structures that are situated below and rostral to the marginal ridge. The marginal ridge has become more prominent than in the preceding stage. The neurohypophysis is elongated and folded, and the adenohypophysis is characteristically expanded. The slender adenohypophysial stalk is still connected with the epithelium of the pharyngeal roof. The olfactory evagination has become prominent. Drawing made by James F. Didusch.
REPRODUCTIVE SYSTEM
In stage 15 the primordial germ cells are found sparsely distributed among the proliferating cells of the coelomic epithelium (fig. 18-9). The coelomic epithelium provides the framework for the gonad. In growth of the epithelium in stage 16 is sufficiently advanced to produce a crescentic strip of gonadal tissue that is fairly well demarcated from the mesonephros. In stage 17 the gonad has increased in size and is beginning to acquire an oval form. Its surface is still irregular. By stage 18 the gonad has become an elongated oval, distinctly set off from the mesonephros. The surface of the gonad is now smooth. Angiogenesis is commencing. The gonadal framework may begin to show sexual distinction by the presence of testicular cords. The presence of testicular cords in the gonadal blastema at stage 18 and of a tunica albuginea at stage 19 makes possible the determination of sexual differentiation in the male embryo.
Page 232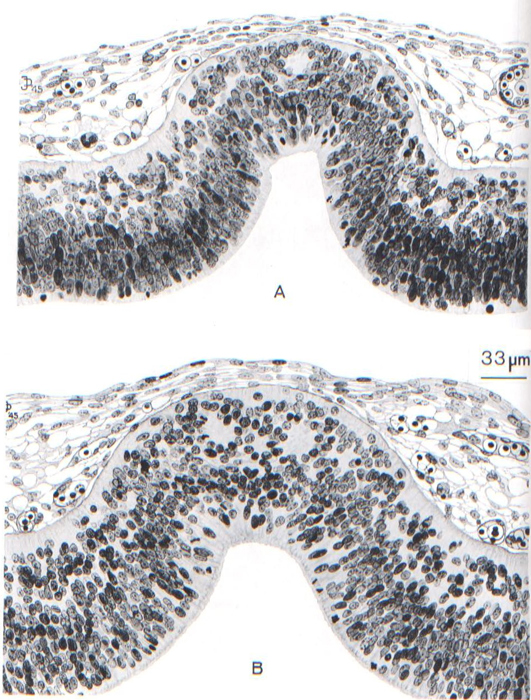
Fig. 18-11. Sections through the roof of the third ventricle of two embryos belonging to stage 18, showing first steps in the formation of the “anterior lobe” of the epiphysis cerebri. Nuclei migrate externally from the ependymal stratum and form a mantle zone having a tendency to follicular arrangement of its cells. These minute follicles are not connected with the lumen of the evagination. The elongation of the epiphyseal evagination into a duct is relatively backward, and follows rather than precedes the formation of the “anterior lobe.” (A) No. 6527, section 11-3-4. (B) No. 7707, section 17-1-5. Drawing made by James F. Didusch.
Page 233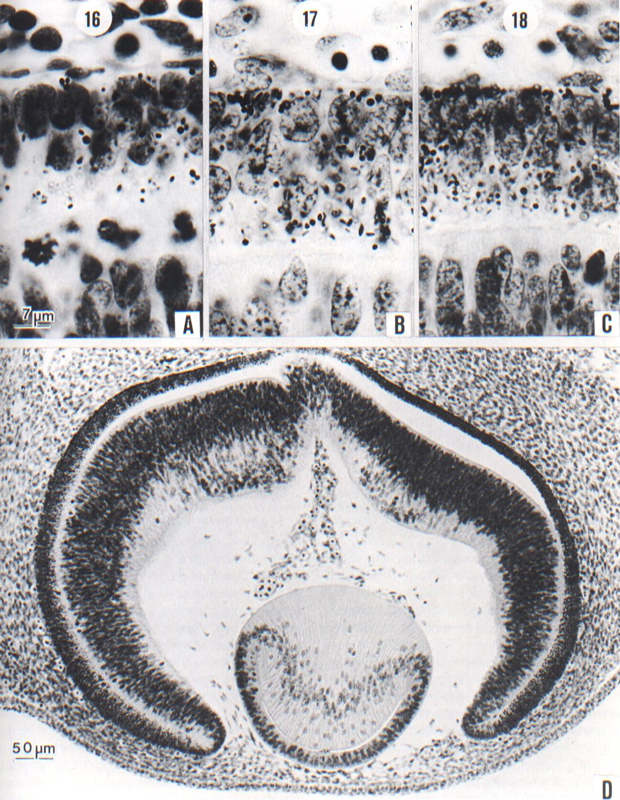
Fig. 18-12. (A-C) The retina at stages 16–18, showing the deposit of pigment granules in the external lamina as seen under a magnification of 1500. In A, only a few distinct granules and some refractive colorless droplets are visible. In B. the granules are more numerous and they show a tendency to cluster at the external surface. In C, a crust of granules is commonly visible on the external surface. In judging the amount of pigment, allowance must be made for section thickness. Angioblasts can be seen on the outer surface of the pigmented layer of the retina. At the bottom of the photographs is seen in each instance the inverted (neural) lamina of the retina. (A) No 6507, 7-1-3, 10 µm. (B) No. 6520, 23-3-5,10 µm. (C) No. 6528, 25-2-3, 8 µm.
(D) Section through an eye typical of stage 18. The migration to form the internal neuroblastic layer of the retina is now widespread. The cavity of the lens vesicle is reduced to a slit, with a corresponding building up of the body of lens fibers. One can see the characteristic distribution of lental nuclei and the fiber lines. It is to be noted that the mesenchyme has begun to invade the interval between lens epithelium and surface epithelium, a prelude to the formation of the cornea. Embryo No. 6527, section 19-3-5.
Page 234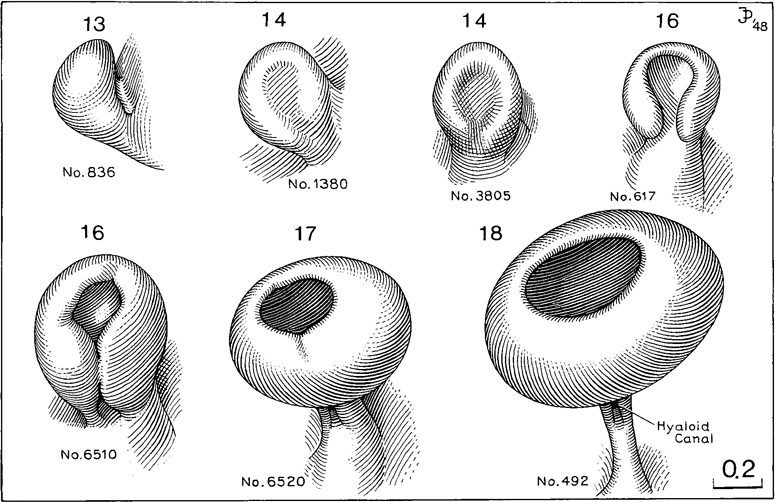
Fig. 18-13. Drawings of reconstructions of the optic vesicle and optic cup at stages 13–18. The drawings show steps in the growth and transformation of the optic vesicle into the optic cup, as well as the retinal fissure and the origin of the hyaloid canal. In the second specimen of stage 16, the rim of the optic cup can be seen to be polygonal.
A precise area of the coelomic epithelium, at the rostral end of the mesonephros, becomes thicker and invaginated to form the paramesonephric duct. The site of its infolding becomes the abdominal ostium of the uterine tube. In stage 18 the paramesonephric duct makes the principal part of its way down the front of the mesonephros, as a lateral companion to the mesonephric duct (fig. 18-7). The origin and rate of growth and elongation are precisely regulated, so that an additional clue to the level of development is provided. The duct tends to be straight, and its length can be calculated by multiplying the number of transverse sections by the thickness of each section. Alternatively, the length can be obtained by superimposing outlines of sagittal or coronal sections.
The paramesonephric ducts are present in all embryos of stage 18; their length was used by Streeter as an index of development within the stage. Of 35 specimens, a less advanced group of nineteen in which the length of the paramesonephric duct was 0.6 mm or less was distinguished from a more advanced group of sixteen specimens in which it was more than 0.6 mm. Most of the embryos in the first group range from 13 to 16.5 mm, whereas most of those in the second group are 14–17.2 mm in length.
In 34 embryos of stage 18, Streeter found that the length of the paramesonephric duct could be correlated with the level of development of other parts of the embryo. For example, in embryos in which only one semicircular duct had been pinched off in the membranous labyrinth, the paramesonephric duct ranged from 0.2 to 0.4 mm in length. Where two semicircular ducts were present, the range was 0.4–0.7 mm. Where three semicircular ducts were present, the range, Page 235 with but a few exceptions, was 0.8–1.1 mm in length (fig. 18-8). Hence, from the status of the paramesonephric duct, the degree of development of an organ as remote as the internal ear can be estimated.
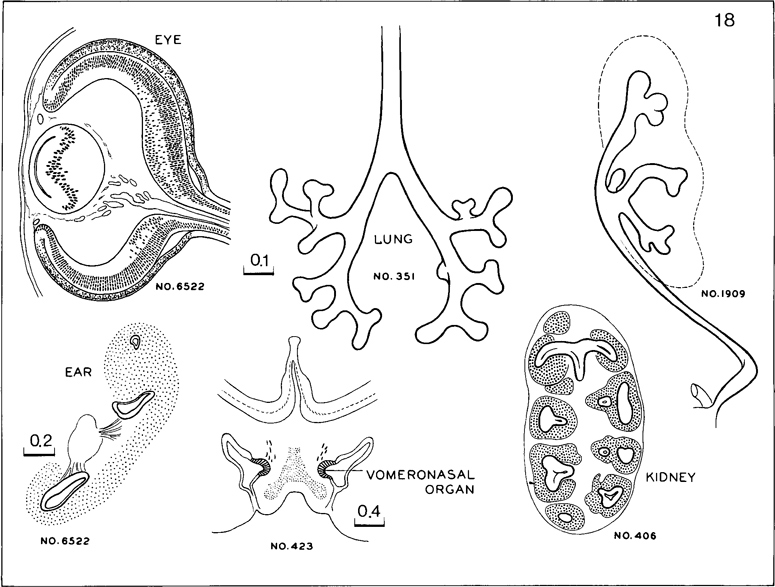
Fig. 18-14. Cluster of features characteristic of stage 18. In the eye the lens vesicle is reduced almost to a slit. The internal neuroblastic layer of the retina is widespread. The inner ear has from one to three semicircular ducts. A distinct vomeronasal organ is present. In the lung, some subsegmental buds are forming. In the kidney, the multiple calices have some sharply marked primordia of collecting tubules. As yet there are no definite glomeruli.
NERVOUS SYSTEM
The most developed area of the brain is the rhombencephalon. The motor nuclei are better organized than the sensory. In the more advanced embryos the choroid plexus of the fourth ventricle begins to be identifiable by the presence of some villi. In the cerebellum, in addition to the inner cerebellar bulge present already in stage 17, an outer swelling appears and represents the future flocculus. Vestibulocerebellar fibers are present in great number at its surface. A clear destination between auditory and optic colliculi in the midbrain as represented by Streeter has not been confirmed. In the diencephalon the neurohypophysis has folded walls. The adenohypophysis, open to the pharynx in stage 17, is now closed off from the pharyngeal cavity. An epithelial stalk, containing a faint lumen, is connected tothe pharyngeal epithelium. The epiphysis, Page 236 representing the “anterior lobe,” is illustrated in figure 18-11. Sections of two specimens (A and B) belonging to the middle third of the embryos of stage 18 are shown. A pineal recess is forming for the first time, and a follicular arrangement of the cells may be encountered in some embryos. This “anterior lobe” of the epiphysis corresponds to Stadium III of Turkewitsch (O'Rahilly, 1968). The rostral part of the diencephalic roof is richly vascularized, and some ingrowth of the epithelial lamina at the level of the telencephalon indicates the first signs of a choroid plexus of the lateral ventricles. Approximately half of the length of the cerebral hemispheres now extends more rostrally than the lamina terminalis. A slight groove is developing in the corpus striatum, which has grown considerably and now reaches as far caudally as the preoptic sulcus. The olfactory bulb is better delimited and, in some embryos, contains an olfactory ventricle.
Eye
Mesenchyme invades the interval between the lens epithelium and the surface ectoderm (as may have already begun in the previous stage), and possibly the posterior epithelium of the cornea (the mesothelium of the anterior chamber) is forming. The cavity of the lens vesicle is becoming obliterated by primary lens fibers.
Reconstructions of the optic vesicle/optic cup at stages 13–18 are shown in figure 18-13.
Ear
The semicircular ducts form from thick epithelial areas of the membranous labyrinth. Adjacent epithelial layers fuse, lose their basement membrane, and disappear (O'Rahilly, 1963). From one to three semicircular ducts are formed during this stage, and the order is anterior, posterior, and lateral. The crus commune is evident from the beginning. The cochlear duct is L-shaped. The mesenchymal stapes (containing the stapedial artery) and the stapedius can be identified, and the bars of pharyngeal arches 1 and 2 may begin to chondrify.
Copyright © 1987 Carnegie Institution of Washington. Reproduced on ehd.org with permission.
